England Launches Faster Cancer Immunotherapy Treatment for Patients
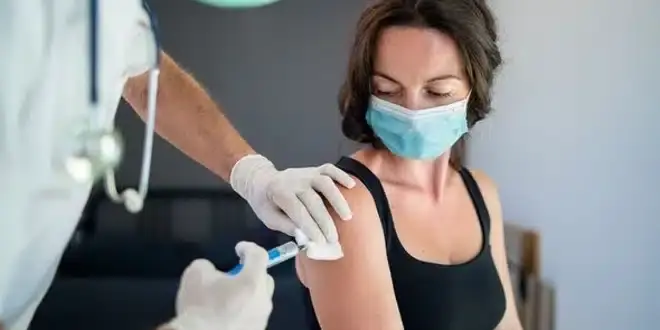
England has recently made significant strides in cancer treatment by becoming the first country in Europe to offer a faster, more convenient form of immunotherapy for cancer patients. This new method is available through the National Health Service (NHS) and involves administering drugs such as atezolizumab (Tecentriq) and nivolumab (Opdivo) via a subcutaneous (under-the-skin) injection.
Faster and More Efficient Cancer Treatment
Traditionally, immunotherapy treatments like atezolizumab and nivolumab were administered intravenously, taking up to an hour. However, this new subcutaneous injection method significantly shortens treatment time to just 3–7 minutes, making it not only more efficient but also more convenient for patients. This advancement allows cancer patients to spend less time in hospitals and receive the same level of clinical care.
Approved for Multiple Types of Cancer
The subcutaneous immunotherapy injection is approved for treating up to 15 different types of cancer, including lung, bladder, breast, liver, kidney, oesophageal, head and neck, and skin cancers. These checkpoint inhibitors work by boosting the immune system’s ability to identify and attack cancer cells, helping the body to fight against the disease more effectively.
Cost-Neutral for the NHS
Thanks to negotiated pricing agreements, the new method is cost-neutral for the NHS, which means that while patients benefit from faster treatments, the system does not incur additional costs. Moreover, the rollout of this innovative method is expected to free up thousands of clinician hours each month, allowing healthcare providers to treat more patients and optimize their workflows.
Proven Effectiveness
Clinical trials such as the IMscin001 study have demonstrated that the subcutaneous version of these drugs is as effective and safe as the traditional intravenous form. This trial showed no significant differences in outcomes between the two methods, proving that the subcutaneous injections maintain the same therapeutic benefits while offering a more efficient delivery method.
A Step Forward in Cancer Treatment Accessibility
This new treatment method represents a major step forward in cancer treatment within the UK, improving accessibility, reducing hospital visits, and enhancing the overall patient experience. As the NHS leads the way in implementing this advanced cancer therapy, it sets the stage for a wider rollout across Europe and beyond.
Sources:
-
NHS England – Information on cancer treatments and their improvements.
-
ClinicalTrials.gov – IMscin001 trial and study results.
-
Medical News Today – Updates on immunotherapy advancements and clinical findings.
This groundbreaking approach to cancer treatment in England marks a major milestone in the ongoing effort to make cancer care more accessible, faster, and more efficient for patients worldwide.